Glioblastoma, an aggressive and often fatal form of brain cancer, has long posed a formidable challenge to doctors and patients alike. Yet, a groundbreaking clinical trial is offering a glimmer of hope, capturing global attention for its potential to revolutionize cancer treatment. A 62-year-old engineer, faced with a grim prognosis, has experienced something extraordinary—his tumour has shrunk significantly in a matter of weeks. This remarkable outcome marks the beginning of a journey that could redefine how we treat one of the most challenging cancers. What makes this approach so promising, and how could it change the future for patients?
Understanding Glioblastoma
Glioblastoma, often referred to as glioblastoma multiforme (GBM), is the most aggressive and common form of primary brain cancer in adults. Originating from glial cells—specifically astrocytes that support nerve cells—this malignancy is notorious for its rapid growth and diffuse infiltration into surrounding brain tissue, making complete surgical removal challenging.
Characteristics and Challenges:
- Aggressiveness: Glioblastomas are classified as grade IV tumors, indicating a high degree of malignancy. They proliferate swiftly and have a propensity to invade adjacent brain regions, complicating treatment efforts.
- Symptoms: Early signs are often nonspecific, including persistent headaches, personality changes, nausea, and symptoms resembling a stroke. As the tumor advances, symptoms can escalate rapidly, potentially leading to unconsciousness.
- Prognosis: The outlook for glioblastoma patients remains dire. Even with aggressive treatment—comprising surgery, radiation, and chemotherapy—the median survival time is approximately 12 to 15 months, with a five-year survival rate of less than 10%.
Current Treatment Modalities:
- Surgical Intervention: The primary approach involves maximal safe resection of the tumor to alleviate symptoms and reduce mass effect. However, due to the tumor’s infiltrative nature, achieving complete removal is often unfeasible.
- Radiation Therapy: Post-surgical radiation aims to destroy residual cancerous cells. This treatment is typically administered over several weeks and is a cornerstone in glioblastoma management.
- Chemotherapy: Temozolomide is the standard chemotherapeutic agent used alongside radiotherapy. It functions by interfering with the tumor’s DNA replication, thereby inhibiting cell division.
Despite the multimodal treatment approach, glioblastomas invariably recur, underscoring the critical need for novel therapeutic strategies. The recent clinical trial at University College London Hospitals NHS Foundation Trust (UCLH) exemplifies such innovation. By delivering targeted radioactive therapy directly into the tumor, this method aims to eradicate cancer cells while preserving healthy brain tissue. Paul Read, the first participant in this trial, described it as a “lifeline,” noting, “I have got nothing to lose and everything to hope for.”
The Innovative Treatment

In a pioneering effort to combat glioblastoma, University College London Hospitals NHS Foundation Trust (UCLH) has initiated a clinical trial exploring a novel treatment approach. This method involves the direct injection of low-level radioactivity into the tumor, aiming to eradicate cancer cells while preserving healthy brain tissue.
The procedure begins with surgeons removing as much of the tumor as possible. Subsequently, a small medical device known as an Ommaya reservoir is implanted under the patient’s scalp, connected to the tumor site via a tube. This reservoir facilitates the direct administration of the radioactive drug ATT001, an iodine-123 labeled PARP inhibitor, into the tumor. The treatment is administered weekly over a period of four to six weeks. The localized delivery ensures that the radioactivity targets cancerous cells specifically, minimizing damage to surrounding healthy tissue.
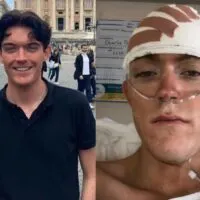
Paul Read, a 62-year-old engineer from Luton, was the first patient to participate in this trial. Diagnosed with glioblastoma in December 2023, he underwent initial treatments, including surgery, radiotherapy, and chemotherapy. However, by July, his tumor had resumed growth. Upon joining the trial, Mr. Read experienced a remarkable 50% reduction in tumor size within weeks. He described the trial as a “lifeline,” stating, “I have got nothing to lose and everything to hope for.” Notably, he reported minimal side effects, aside from slight fatigue.
Dr. Paul Mulholland, the UCLH consultant medical oncologist who designed the trial, expressed optimism about the results, noting, “We’ve just gone through [Paul’s] scan results with him and his end of treatment scan shows a reduction in the tumor, which is really quite remarkable for … .” The trial, known as CITADEL-123, plans to treat up to 40 patients in its initial phase, with future plans to increase the radiation dose and combine the drug with immunotherapy to enhance the body’s immune response against cancer.
Paul Read’s Journey

Image Credit: Twitter @Independent
Paul Read’s battle with glioblastoma began when he was diagnosed with this aggressive brain tumor in December of the previous year. His journey through the standard treatment protocol of surgery, radiotherapy, and chemotherapy seemed all too familiar for glioblastoma patients, characterized by brief respites from the disease before inevitable regrowth. By July, despite the aggressive treatment, his tumor had started growing again, a common and disheartening phase for many battling this condition.
Determined to fight the disease, Paul enrolled as the first patient in a pioneering clinical trial at University College London Hospitals NHS Foundation Trust (UCLH), seeking a new kind of treatment that promised more than just temporary control. “I am more than happy – even if it doesn’t benefit me, it may benefit someone else down the line,” Paul stated, reflecting a selfless perspective on his participation.
The innovative treatment involved injecting a radioactive drug directly into his tumor, a process facilitated by the implantation of a small medical device called an Ommaya reservoir under his scalp. This device connected directly to the tumor, allowing for precise delivery of the treatment intended to minimize harm to healthy brain tissue. Remarkably, within just a few weeks of starting the trial, Paul observed a significant reduction in his tumor size, halved from its original state. “This trial was a lifeline,” he remarked, underscoring the personal significance of the experimental approach not just as a treatment but as a beacon of hope.
Paul’s response to the treatment was not just physical but also emotional, as he experienced minimal side effects, a stark contrast to the often debilitating impacts of conventional treatments. “I am feeling very good,” he noted, which highlighted the dual benefits of the trial—efficacy in treating the tumor and maintaining quality of life.
Expert Insights

The clinical trial at University College London Hospitals NHS Foundation Trust (UCLH) has not only showcased remarkable patient outcomes but also garnered significant attention from medical experts in the field. Dr. Paul Mulholland, the oncologist and chief investigator of the trial, expressed optimism about the potential of this new treatment to change the landscape of brain cancer therapy. “We have to aim to cure this disease,” he asserted, highlighting the unique opportunity provided by the localized nature of glioblastoma, which does not typically metastasize beyond the brain.
Dr. Mulholland further explained the scientific rationale behind the approach, “Primary brain tumors do not metastasize around the body and generally stay in the same location in the brain. It doesn’t spread to the rest of the body, so using a targeted – directly into the tumor – approach makes sense.” This targeted approach allows for high doses of therapeutic agents right at the site of the tumor, maximizing the impact on cancer cells while sparing healthy tissue.
The optimism is also shared by other experts in the field. Dr. Simon Newman, chief scientific officer at The Brain Tumour Charity, commented on the trial’s implications, “These tumors are notoriously difficult to treat, and research into immunotherapy has had mixed results due to the tumor’s ability to hide from the immune system. However, we are encouraged by the findings from this study as there is an urgent need for new approaches to monitor and treat this devastating disease.”
Hope for the Future
The promising results of the clinical trial at University College London Hospitals NHS Foundation Trust (UCLH) have instilled a new sense of hope among patients, families, and clinicians alike in the fight against glioblastoma. This hope is not only rooted in the current successes but also in the potential for future advancements that this research may enable.
As Dr. Paul Mulholland, the trailblazing oncologist behind the trial, explains, “The dose of radiation will be increased throughout the trial and the plan is then to combine the drug with an immunotherapy—which trains the body’s own immune system to kill cancer.” This forward-looking approach aims not only to refine the treatment but to possibly establish a new standard of care that could dramatically improve survival rates and quality of life for patients with glioblastoma.
Furthermore, the enthusiastic reception and support from the broader medical and research community highlight the importance of continued investment in innovative cancer treatments. Dr. Simon Newman, chief scientific officer at The Brain Tumour Charity, reflected on the broader implications: “Immunotherapies have shown progress in other cancer types, and we hope to see similar advancements for brain tumors. We are pleased to see progress in this area and look forward to following this work as it advances to larger clinical trials.”
This hope is not just clinical but deeply personal for those affected. As Paul Read, a participant in the trial, poignantly shares, “It will be wonderful if this treatment helps me, and if it doesn’t, it doesn’t. I am more than happy—even if it doesn’t benefit me, it may benefit someone else down the line.” His sentiment encapsulates the dual aspirations of the trial: to find a cure and to contribute to a legacy of improved outcomes for future generations. This trial, therefore, is not just about treating a disease but about changing the narrative of glioblastoma for patients worldwide.
A New Dawn in Glioblastoma Treatment
The innovative clinical trial led by University College London Hospitals NHS Foundation Trust marks a significant advancement in the treatment of glioblastoma. By successfully reducing Paul Read’s tumor by half through targeted radioactive therapy, this trial offers a beacon of hope not just for him but for all glioblastoma patients. The potential to change the standard treatment approach for this aggressive cancer could improve survival rates and quality of life for many.
As we look to the future, the integration of this therapy with immunotherapy promises even greater strides in treating not only glioblastoma but potentially other localized cancers. The ongoing research and dedication of medical professionals and researchers are vital to turning these possibilities into realities.
This trial not only signifies a breakthrough in medical treatment but also embodies the relentless pursuit of better outcomes for patients facing dire prognoses. It reinforces the importance of continuous innovation and optimism in the face of one of the toughest medical challenges.